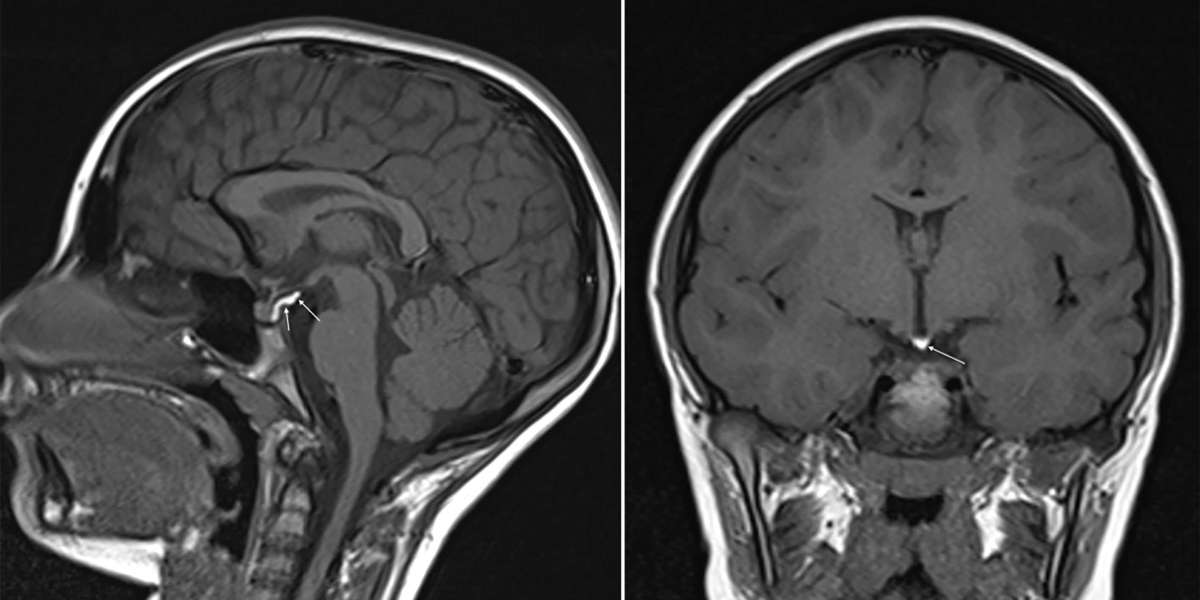
Multiple System Atrophy (MSA) is a rare neurodegenerative disorder characterized by autonomic dysfunction, parkinsonism, and cerebellar ataxia. Therapeutic approaches focus on symptom management, slowing disease progression, and improving patient quality of life. Key products include dopamine agonists, botulinum toxin injections for autonomic symptoms, and investigational agents targeting alpha-synuclein aggregation. Multiple System Atrophy (MSA) Market Advantages of these therapies lie in their potential to reduce motor impairment, stabilize cardiovascular functions, and offer personalized regimens based on disease stage. The increasing prevalence of MSA, coupled with growing demand for early and accurate diagnostics, underscores the need for enhanced imaging tools and biomarker panels. Furthermore, rising awareness among clinicians and patients about disease monitoring has fueled investment in research and development. Robust clinical trials and strategic collaborations among biotech companies aim to expand the therapeutic pipeline.
The Multiple System Atrophy (MSA) Market is estimated to be valued at USD 155.1 Mn in 2025 and is expected to reach USD 213.9 Mn by 2032, growing at a compound annual growth rate (CAGR) of 4.7% from 2025 to 2032.
Key Takeaways
Key players operating in the Multiple System Atrophy (MSA) Market are Chelsea Therapeutics International, Ltd.; Biohaven Pharmaceuticals, Inc.; Sumitomo Dainippon Pharma Co., Ltd.; AstraZeneca plc; and Theravance Biopharma, Inc. These market players drive competitive dynamics by exploring novel compounds and forging licensing agreements. Their combined efforts influence market share distribution and support comprehensive market analysis through ongoing clinical trials and partnership announcements.
The market presents significant market opportunities in gene therapy platforms, precision medicine, and digital health solutions for MSA. Unmet medical needs and limited treatment options create an attractive environment for investment and business growth. Advances in patient registries and real-world evidence generation enhance market research capabilities, refining market forecasts and segmentation for targeted product launches. Strategic alliances between emerging biotechs and established pharmaceutical companies further amplify commercialization potential.
Advancements in biomarker-based diagnostics are reshaping market trends by enabling earlier and more accurate detection of MSA. Cutting-edge assays for cerebrospinal fluid proteins, neuroimaging modalities, and wearable sensor technologies provide deeper market insights and improve patient stratification. These innovations not only inform clinical decision-making but also drive market dynamics by accelerating trial enrollment and regulatory approval processes.
Market drivers
One of the primary market drivers is the rising prevalence of neurodegenerative diseases and growing geriatric population worldwide. As life expectancy increases, the incidence of conditions such as MSA is projected to rise, fueling demand for effective therapies. Additionally, government initiatives and public funding for rare disease research have intensified, reducing market restraints related to RD costs. Improved reimbursement policies and orphan drug incentives further stimulate pharmaceutical companies to develop novel treatments, accelerating product pipelines. Continued advancements in clinical trial design and collaborative research networks are expected to support sustained market growth over the forecast period.
Current Challenges in the Multiple System Atrophy (MSA)
Multiple System Atrophy presents a series of pressing market challenges driven by diagnostic complexity and limited therapeutic options. Early-stage symptoms overlap with Parkinson’s disease and other movement disorders, making accurate diagnosis a key market restraint. This diagnostic ambiguity often delays intervention and impedes clinical trial enrollment, hampering overall business growth in the MSA field. Additionally, the small patient pool leads to high per-patient development costs, posing financing hurdles for emerging market companies and reducing overall market attractiveness.
Research and development efforts face strict regulatory scrutiny, intensifying time-to-market and adding to compliance burdens. Limited understanding of disease drivers and underlying pathology also restricts the pool of viable drug targets, which dampens market growth prospects. Finally, pricing pressures and reimbursement uncertainties in major healthcare systems complicate market access, forcing stakeholders to refine market access strategies and demonstrate real-world value through health economics and outcomes research. Amid these market dynamics, collaboration between academic centers, contract research organizations, and industry partners is crucial to surmount these barriers and unlock new market opportunities.
SWOT Analysis
Strength: Robust clinical expertise and advanced biomarker research have improved diagnostic accuracy for MSA. Ongoing collaborations between research institutes and contract research organizations enhance trial design, bolstering market insights.
Weakness: Limited patient population increases development costs and challenges scalability of clinical trials. Gaps in understanding regional disease prevalence create obstacles for global trial enrollment and market segmentation.
Opportunity: Advances in precision medicine and imaging technologies offer potential for targeted therapeutics, expanding market scope. Growing interest in neuroprotective strategies presents new market opportunities for disease-modifying agents.
Threats: Stringent regulatory pathways and high attrition rates in neurodegenerative research heighten development risks. Competition from parallel research in Parkinson’s and other movement disorders may divert funding and trial resources.
Geographical Regions by Revenue Concentration
North America currently holds the largest concentration of market revenue for Multiple System Atrophy therapeutic development. The region’s established reimbursement framework and well-funded healthcare infrastructure support extensive clinical trial activity. Strong networks of specialized movement disorder centers facilitate rapid patient recruitment, boosting market share in disease management and drug development efforts. Furthermore, the U.S. Food and Drug Administration’s accelerated approval pathways for rare neurological conditions have attracted significant RD investments, reinforcing the region’s leading position.
Europe follows closely, driven by centralized health technology assessment bodies and pan-European trial networks. Key countries such as Germany, France, and the U.K. contribute substantial market revenue due to proactive screening programs and robust patient registries. These systems enhance disease awareness and generate critical epidemiological data, supporting both market research and market growth strategies. Amid this environment, collaborations with regional regulatory bodies have streamlined protocol approvals and fostered an environment conducive to innovative therapy development.
Fastest-Growing Region for MSA Market
The Asia-Pacific region is emerging as the fastest-growing market for Multiple System Atrophy therapeutics. Rapidly expanding healthcare expenditures and increasing investments in neurological research are pivotal market drivers. Countries such as Japan, South Korea, and China are bolstering infrastructure for rare disease trials, setting up state-of-the-art movement disorder clinics and biobank networks. These initiatives enhance clinical trial readiness and accelerate patient enrollment.
Moreover, growing government support for orphan drug development and favorable pricing policies are fueling market growth. Collaborative ventures between local biotech firms and global market players are driving technology transfers and capacity building. Additionally, rising awareness among healthcare professionals and increased availability of advanced imaging modalities are improving early diagnosis rates, which in turn heightens demand for novel interventions. With ongoing expansion of contract research organizations and dedicated research grants, the Asia-Pacific region is poised to lead in market forecast contributions and overall market growth trajectories for MSA therapeutics.
Get this Report in Japanese Language: 多系統萎縮症(MSA)市場
Get this Report in Korean Language: 다중계통위축증(MSA)시장
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)