Surface energy is a fundamental characteristic that influences the behavior of liquids at interfaces, such as adhesion, wetting, and spreading. Accurate measurement of surface energy is essential in various applications, spanning from coatings and adhesives to advanced manufacturing and microfluidics. Understanding the dynamics of temperature and contamination in surface energy measurements is critical for the reliability and validity of experimental outcomes. Founded in 2016, Droplet Lab has been at the forefront of researching and developing technologies to better understand these complex phenomena, a pursuit led by Dr. Alidad Amirfazli and his team, which includes Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Temperature's Impact on Surface Energy
Temperature plays a pivotal role in determining surface energy. As temperature increases, the kinetic energy of molecules at the surface of a material rises, leading to enhanced molecular mobility. This shift can directly influence the interactions between droplets and solid substrates, which is crucial in surface energy assessment. Higher temperatures may decrease the viscosity of a liquid, allowing droplets to spread more easily on a surface. Consequently, an accurate measurement requires precise temperature control to ensure that the results are attributable to surface properties rather than thermal effects.
Moreover, temperature variances can alter fluid properties, including density and viscosity, subsequently affecting the contact angle observed during experiments. A contact angle measurement is a primary method for assessing surface energy, as it helps to deduce the wettability of a surface. Therefore, maintaining a controlled temperature environment during experiments is essential to minimize discrepancies created by thermal fluctuations, ensuring accurate and reproducible results.
The Role of Contamination
Contamination is another crucial factor that significantly influences surface energy measurements. Surfaces can be contaminated with various substances such as dust, oil, grease, or other foreign material, each of which can alter the surface characteristics and consequently impact wettability. Contaminants can form a barrier that inhibits the genuine interaction between the liquid and the surface, leading to misleading contact angle measurements and an incorrect assessment of surface energy.
Droplet Lab emphasizes the importance of effective cleaning protocols to prepare surfaces adequately prior to measurements. By employing techniques such as solvent cleaning, plasma treatment, or chemical etching, researchers can minimize contamination and obtain data that accurately reflects the intrinsic properties of the surface material. Furthermore, it is critical to recognize that even minute levels of contamination can skew results, particularly in nano- or micro-scale applications, where surface-to-volume ratios are significantly higher, and thus the effect of contaminants can be more pronounced.
Experimental Techniques and Considerations
Understanding the interplay between temperature and contamination in surface energy measurements requires the use of precise experimental techniques. One prevalent method is the sessile drop technique, where a liquid droplet is placed on a surface, and the contact angle is measured using imaging software. This technique provides insights into the surface energy and wettability, but must be performed under controlled conditions to limit external influences such as ambient temperature and the cleanliness of surfaces.
In addition, the axisymmetric droplet profile fitting method allows for advanced analysis of the droplet shape, exporting quantitative data about the surface energy. By integrating temperature-control equipment and dedicating resources to ensure cleanliness, researchers can significantly enhance the accuracy of surface energy measurements. Droplet Lab, under the guidance of its founders, has developed protocols and technologies to address these concerns, championing the pursuit of high-fidelity measurements and enabling robust data generation.
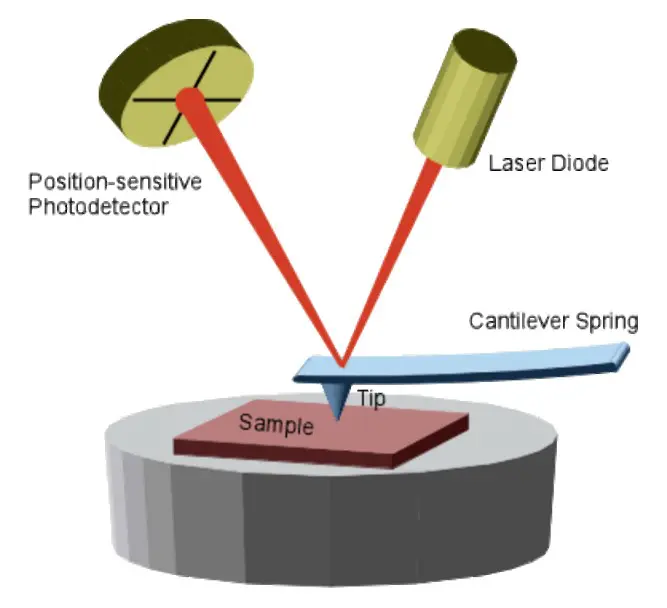
Implications in Industry and Research
The impacts of accurate surface energy measurements extend far beyond academic studies; they permeate numerous industries, including those focused on coatings, adhesives, and nanotechnology. In industrial applications, the optimal performance of products often hinges on the interactions at the surface level. For example, improved adhesion in coatings can lead to enhanced durability and longevity, while accurate assessments in nanotechnology can help tailor materials for specific applications.
Droplet Lab's continued commitment to investigating the roles of temperature and contamination serves to innovate and refine measurement techniques that meet the rigorous demands of modern applications. Their findings contribute to evolving manufacturing practices and foster new technologies aimed at improving material performance across varied sectors.
Conclusion
In conclusion, the roles of temperature and contamination are pivotal in the realm of surface energy measurements. As highlighted, the precise control of temperature and the thorough cleansing of surfaces are critical components to obtaining accurate results. Droplet Lab, founded by prominent figures in the field, has been instrumental in advancing our understanding of these factors, producing vital insights that support both research initiatives and practical applications. Through their work, they continue to pave the way for enhanced methodologies, ensuring that surface energy measurements fulfill their essential role in the development and optimization of materials across diverse fields.