"Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Size And Forecast by 2031
The market scope spans multiple applications, reflecting its adaptability and relevance in today’s industries. A detailed evaluation of industry statistics reveals upward trends in adoption, supported by evolving consumer preferences and policy incentives. This comprehensive analysis underscores the importance of monitoring industry trends to stay competitive in this dynamic space.
Key growth drivers include advancements in technology, increasing consumer awareness, and favorable government policies. Despite these positive indicators, the industry faces challenges such as fluctuating supply chains and regulatory hurdles, which may influence short-term growth. Companies leveraging adaptive strategies are poised to maintain a competitive edge as leaders in the market.
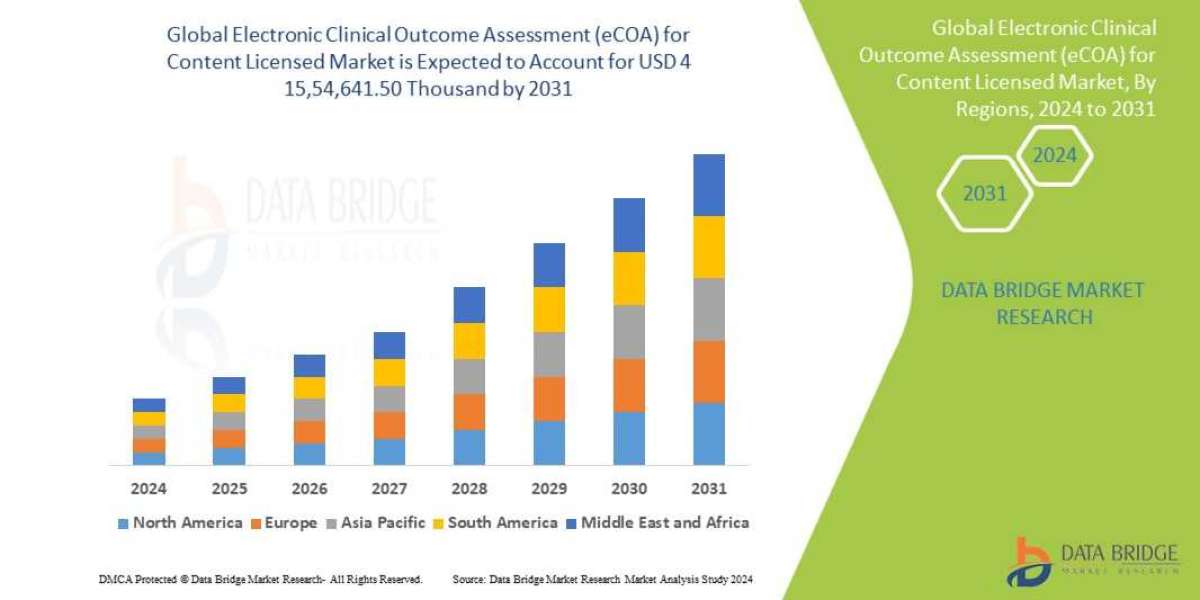
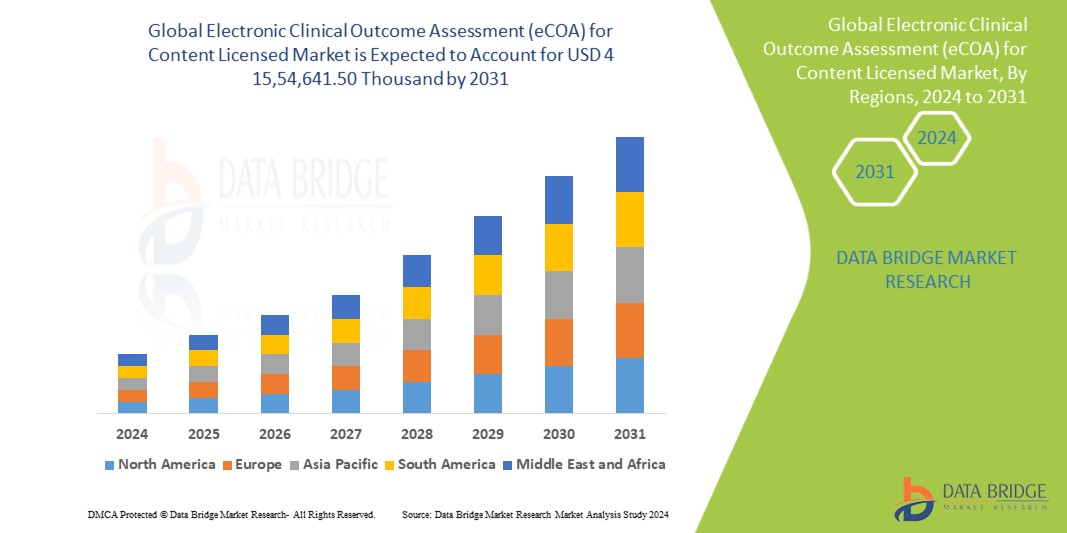
Data Bridge Market Research analyses that the Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market which was USD 474708.5 Billion in 2023 is expected to reach USD 1554641.5Thousand by 2031 and is expected to undergo a CAGR of 14.40% during the forecast period of 2023 to 2031
Get a Sample PDF of Report - https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Which are the top companies operating in the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market?
The global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market study presents a detailed analysis of the industry, focusing on key trends, market dynamics, and the competitive landscape. It highlights leading companies in the market, examining their strategies and contributions to market share. Additionally, the report offers insights into the Top 10 Companies in Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market in the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, including their business strategies, financial performance, and overall market position.
**Segments**
- **By Type**: The market for electronic clinical outcome assessment (eCOA) content licensed can be segmented by type into Patient-Reported Outcome (PRO), Clinician-Reported Outcome (ClinRO), Observer-Reported Outcome (ObsRO), and Performance Outcome (PerfO). Each type caters to specific needs within the healthcare and clinical research sectors, offering a range of options for measuring outcomes and capturing data.
- **By End-User**: The end-user segmentation involves categorizing the market based on the entities that utilize eCOA content licensed solutions. This can include pharmaceutical companies, contract research organizations (CROs), hospitals, academic research institutes, and other healthcare providers. Different end-users have varying needs and requirements when it comes to electronic clinical outcome assessments, thereby driving the demand for specialized solutions.
- **By Application**: Another crucial segment is the application of eCOA content licensed solutions. Applications can range from clinical trials and research studies to real-world evidence collection and patient monitoring. The versatility of eCOA technologies allows for their adaptation across various healthcare settings, making them a valuable asset in improving patient outcomes and streamlining data collection processes.
**Market Players**
- **ERT Clinical**
- **Signant Health**
- **IQVIA**
- **ERT Clinical**
- **OmniComm Systems, Inc.**
- **Advarra**
- **Medidata Solutions**
- **Parexel International Corporation**
- **BioClinica**
- **CRF Health**
- **ERT Clinical**
These market players are at the forefront of the global electronic clinical outcome assessment (eCOA) for content licensed market, offering innovative solutions and services to cater to the evolving needs of the healthcare industry. Through strategic partnerships, product development, and focus on customer satisfaction, these companies are driving the growth and adoption of eCOA technologies worldwide.
[https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outThe market for electronic clinical outcome assessment (eCOA) content licensed is witnessing significant growth and innovation driven by the diverse segments it caters to. The segmentation by type into Patient-Reported Outcome (PRO), Clinician-Reported Outcome (ClinRO), Observer-Reported Outcome (ObsRO), and Performance Outcome (PerfO) allows for a nuanced approach in measuring outcomes and capturing data in the healthcare and clinical research sectors. Each type serves a specific purpose and offers tailored solutions to meet the varied needs of stakeholders in the industry. This segmentation strategy enables organizations to choose the most appropriate eCOA content licensed solution based on their requirements, thereby enhancing the efficiency and effectiveness of clinical assessments and data collection processes.
When considering the end-user segmentation of the eCOA market, it becomes evident that different entities such as pharmaceutical companies, contract research organizations (CROs), hospitals, academic research institutes, and healthcare providers have distinct needs and preferences when it comes to utilizing eCOA content licensed solutions. These end-users play a crucial role in driving the demand for specialized eCOA technologies that can address their specific challenges and requirements. As a result, market players need to develop tailored solutions and services to cater to the diverse needs of each end-user segment effectively.
The segmentation by application further highlights the versatility and adaptability of eCOA content licensed solutions across various healthcare settings. From clinical trials and research studies to real-world evidence collection and patient monitoring, the applications of eCOA technologies are wide-ranging and impactful. By offering a comprehensive suite of applications, eCOA providers can address a broad spectrum of healthcare needs, thereby establishing themselves as essential partners in improving patient outcomes and enhancing data collection practices in both clinical and real-world settings.
In terms of market players, companies such as ERT Clinical, Signant Health, IQVIA, OmniComm Systems, Inc., Advarra, Medidata Solutions, Parexel International Corporation, BioClinica, and CRF Health are**Market Players:**
- Oracle (U.S.)
- IBM Corporation (U.S.)
- Dassault Systemes (France)
- Parexel International Corporation (U.S.)
- ERT Clinical (U.S.)
- eClinical Solutions LLC (U.S.)
- ArisGlobal (U.S.)
- Clinical Ink (U.S.)
- Kayentis (France)
- Anju Software, Inc. (U.S.)
- Signant Health (U.S.)
- WIRB-Copernicus Group (U.S.)
- YPrime LLC (U.S.)
- Bioclinica (U.S.)
The global market for electronic clinical outcome assessment (eCOA) content licensed is experiencing significant expansion and innovation due to its diverse market segments and the emergence of key market players providing cutting-edge solutions. The segmentation by type into various outcome measures such as PRO, ClinRO, ObsRO, and PerfO enables tailored approaches to data collection and outcome assessment, thereby meeting specific requirements within the healthcare and clinical research domains. This segmented approach allows organizations to select the most suitable eCOA solutions based on their needs, ultimately enhancing the accuracy and efficacy of clinical assessments and data capture.
In terms of end-user segmentation, different entities including pharmaceutical companies, CROs, hospitals, academic research institutes, and healthcare providers have distinct demands for eCOA content licensed solutions. The varied requirements of these end-users act as drivers for specialized eCOA technologies that can address their specific challenges effectively.
Explore Further Details about This Research Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Report https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Why B2B Companies Worldwide Choose Us for Revenue Growth and Sustainability
- Gain a clear understanding of the Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market, its operations, and stages in the value chain.
- Explore the current market scenario and assess future growth potential throughout the forecast period.
- Strategize effectively for marketing, market entry, expansion, and business plans by analyzing growth factors and buyer behavior.
- Stay ahead of competitors by studying their business models, strategies, and prospects.
- Make data-driven decisions with access to comprehensive primary and secondary research.
Key Insights from the Global Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market :
- Comprehensive Market Overview: A detailed examination of the global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market.
- Industry Trends and Projections: Analysis of historical data (2015 onward) and future growth forecasts, including compound annual growth rates (CAGRs).
- Emerging Opportunities: Identification of new market prospects and targeted marketing strategies.
- Focus on RD: Insights into demand for new product launches and innovative applications.
- Leading Player Profiles: Detailed profiles of major market participants.
- Market Composition: Analysis of dynamic molecule types, targets, and key resources.
- Revenue Growth: Examination of global market revenue, segmented by key players and product categories.
- Commercial Opportunities: Analysis of sales trends, licensing deals, and co-development opportunities.
Regional Insights and Language Accessibility
- North America: United States, Canada, Mexico
- Europe: Germany, France, UK, Russia, Italy
- Asia-Pacific: China, Japan, Korea, India, Southeast Asia
- South America: Brazil, Argentina, Colombia, and others
- Middle East and Africa: Saudi Arabia, UAE, Egypt, Nigeria, South Africa
Understanding market trends at a regional level is crucial for effective decision-making. Our reports cater to diverse audiences by offering localized analyses in multiple regional languages. These reports provide tailored insights for specific regions, enabling businesses and stakeholders to access relevant information for informed strategies. By bridging communication gaps, we empower regional markets to thrive and grow. Access our reports in your preferred language for a personalized understanding of industry dynamics.
Japanese : https://www.databridgemarketresearch.com/jp/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Chinese : https://www.databridgemarketresearch.com/zh/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Arabic : https://www.databridgemarketresearch.com/ar/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Portuguese : https://www.databridgemarketresearch.com/pt/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
German : https://www.databridgemarketresearch.com/de/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
French : https://www.databridgemarketresearch.com/fr/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Spanish : https://www.databridgemarketresearch.com/es/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Korean : https://www.databridgemarketresearch.com/ko/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Russian : https://www.databridgemarketresearch.com/ru/reports/global-electronic-clinical-outcome-assessment-ecoa-for-content-licensed-market
Data Bridge Market Research:
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 975