RNA Pull Down is a molecular biology method used to investigate RNA-protein interactions. It enables the isolation and identification of proteins that bind to specific RNA molecules. This technique has emerged as a cornerstone in molecular biology, facilitating the understanding of cellular processes at a molecular level.
Basic Principle of RNA Pull Down
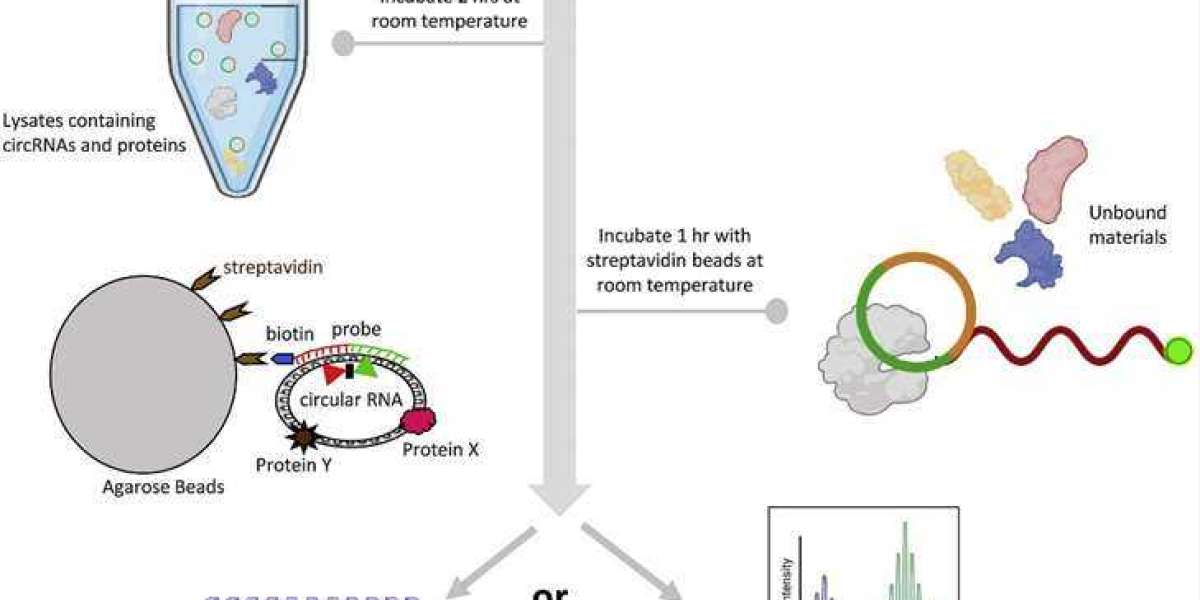
The basic principle of RNA Pull Down involves the isolation of RNA-binding proteins (RBPs) that interact with a specific RNA of interest. This technique utilizes biotinylated RNA probes that are complementary to the target RNA sequence. These probes are incubated with cellular lysates, allowing the RBPs to bind to the RNA of interest. The RNA-protein complexes are then captured using streptavidin-coated beads, and subsequently, the proteins bound to the RNA are eluted, identified, and analyzed. This method helps researchers understand which proteins interact with a particular RNA molecule, shedding light on the RNA's functional roles.
Materials and Reagents Required
Biotinylated RNA Probes: Synthesize or purchase biotinylated RNA probes specific to your RNA of interest. These probes should be high-quality and designed to ensure specificity.
Cell Lysates: Prepare cell lysates from the cells or tissues of interest. These lysates should contain the proteins you want to study. Be sure to include protease and RNase inhibitors to maintain protein and RNA integrity.
Streptavidin Beads: Obtain streptavidin-coated magnetic or agarose beads. These beads will be used to capture the RNA-protein complexes.
Buffer Solutions: Prepare buffers for RNA Pull Down, including binding buffer (for incubating RNA probes with lysates), washing buffer (for washing away unbound proteins), and elution buffer (for releasing RNA-bound proteins).
Protease and RNase Inhibitors: Add protease inhibitors to protect proteins from degradation and RNase inhibitors to preserve RNA integrity during the experiment.
Magnetic Rack or Centrifuge: You'll need a magnetic rack for magnetic beads or a centrifuge for agarose beads to separate the RNA-protein complexes from the supernatant.
SDS-PAGE Gel and Electrophoresis Setup: You'll need these for analyzing and visualizing the isolated proteins.
Western Blotting Materials (if needed): This includes antibodies specific to the RBPs you want to detect and the necessary reagents for Western blotting analysis.
Learn other articles:
cross linking mass spectrometry
Step-by-Step Protocol for RNA Pull Down
a) Prepare Cell Lysates:
Harvest and lyse cells or tissues of interest using a suitable lysis buffer containing protease and RNase inhibitors.
Centrifuge the lysate to remove cellular debris and obtain the soluble protein fraction.
b) Biotinylated RNA Probe Incubation:
Incubate the biotinylated RNA probes with the cell lysates in a binding buffer. This step allows RBPs to bind to the RNA of interest.
Ensure the incubation is performed at an appropriate temperature and for a sufficient duration to allow complex formation.
c) Capture RNA-Protein Complexes:
Add streptavidin-coated beads to the RNA-protein mixture and incubate. The biotin on the RNA probes will bind to the streptavidin on the beads, capturing the RNA-protein complexes.
d) Wash Steps:
Wash the beads several times with a washing buffer to remove unbound proteins and reduce background noise.
e) Elution of RNA-Bound Proteins:
Elute the RNA-bound proteins from the beads using an elution buffer.
Collect the eluted proteins for downstream analysis.
f) Analysis:
Analyze the eluted proteins using techniques such as SDS-PAGE and Western blotting to identify and characterize the interacting RBPs.
Alternatively, mass spectrometry can be used for a comprehensive analysis of the protein composition.
g) Data Interpretation:
Analyze the results to identify and validate RNA-protein interactions. Compare the proteins isolated in the experiment with controls.
h) Further Characterization (Optional):
Perform additional experiments or validations to confirm the functional significance of the identified RNA-protein interactions.
Designing RNA Probes for RNA Pull Down
Designing RNA probes is a critical step in the RNA Pull Down assay, as the quality and specificity of these probes profoundly influence the success and accuracy of the experiment. Here's why designing specific and effective RNA probes is crucial:
- Specificity:
RNA Pull Down aims to capture RNA-binding proteins that interact with a particular RNA molecule. Therefore, the RNA probes must be highly specific to the target RNA of interest.
Non-specific probes may lead to the capture of unrelated proteins, resulting in noisy data and incorrect conclusions.
Specific probes ensure that the identified proteins are truly associated with the RNA of interest, enhancing the reliability of the experiment.
- Effectiveness:
Effective RNA probes should have a high affinity for the target RNA sequence. This ensures that the RNA-protein interactions are efficiently captured.
Ineffectual probes with weak binding affinity may fail to pull down the associated proteins, leading to false-negative results.
Effective probes optimize the chances of successfully identifying RNA-binding proteins and quantifying their binding.
Choosing the target RNA sequences that the RNA probes will mimic is a critical decision in the design process. Here's how to go about it:
1. Knowledge of RNA of Interest:
Start by thoroughly understanding the RNA molecule you wish to study. This could be a known regulatory RNA, a long non-coding RNA, or any RNA with suspected roles in specific cellular processes.
Gather information about its sequence, structure, and known or predicted binding partners.
2. Identifying Binding Sites:
Identify the regions or specific sequences within the RNA that are known or suspected to interact with RNA-binding proteins.
Utilize existing literature, databases, or bioinformatics tools to pinpoint potential binding sites.
3. Probe Design Parameters:
Design the RNA probes to mimic the identified binding sites or regions. These probes should be of sufficient length to encompass the binding site and should be designed with the following considerations:
Biotinylation: Incorporate biotin into the probes to facilitate their capture during the Pull Down process.
Secondary Structure: Account for potential secondary structures within the RNA when designing the probes to ensure they accurately mimic the target RNA's structure.
Control Probes: Include control probes, such as scrambled sequences or non-binding sequences, to serve as negative controls in the experiment.
4. Validation:
Prior to conducting RNA Pull Down experiments, it is advisable to validate the designed RNA probes in vitro to confirm their ability to bind the target RNA and associated proteins.